Rotavirus Updates
May 25, 2012
Rwanda introduces rotavirus vaccines to save children from deadly diarrhea Rollout of rotavirus vaccines across Africa continues
Rwanda today became the
third GAVI-eligible African country to introduce vaccines against
rotavirus, the leading cause of severe and deadly diarrhea in young
children. Rotavirus is responsible for
close to 3,500 deaths in children
under five years of age every year in Rwanda, which amounts to
8.8% of all
under five deaths nationwide. Vaccines are the best way to protect children
in Rwanda and the rest of the world from severe rotavirus disease and the
deadly dehydrating diarrhea that it causes. Now that Rwandan children will
have access to life-saving rotavirus vaccines, much of this death and
suffering can be prevented. "Introducing rotavirus vaccines gives us the opportunity to save many lives
and to reduce the impact of illness on our hospitals and within our
communities," said Maurice Gatera, EPI Manager, Rwanda Ministry of Health. Burden of rotavirus in Africa
Children in Africa bear the heaviest burden of rotavirus
disease—approximately 40 percent of children hospitalized for diarrhea test
positive for rotavirus.
Nearly 50 percent of the more than 450,000 deaths
annually from rotavirus occur in Africa where access to treatment for the
deadly dehydrating diarrhea caused by rotavirus is limited or unavailable.
Rotavirus surveillance was initiated in Rwanda in October 2010 to track the
burden of rotavirus disease. The Ministry of Health is working to strengthen
the surveillance system to effectively monitor the impact of vaccine
introduction on child deaths and hospitalizations. Expected impact of rotavirus vaccines in Rwanda
In April 2009, Rwanda became the
first developing country to introduce pneumococcal vaccines into its routine immunization program, which led to a
significant decline in respiratory infections. Ministry of Health officials
expect the introduction of rotavirus vaccines have a
similar dramatic impact
on the incidence of severe infant diarrhea as it has in other countries
already using the vaccine. GAVI support for rotavirus vaccines in Africa
With widespread use of rotavirus vaccines, we can drastically reduce the
numbers of young children who are hospitalized or die from severe diarrhea
and greatly improve child health in the developing world. Rwanda is the
third African country to utilize GAVI support for rotavirus vaccine
introduction after Sudan (July 2011) and Ghana (April 2102). This brings to
seven the total number of GAVI-eligible countries that have introduced
rotavirus vaccines. GAVI plans to vaccinate more than 50 million children in
40 of the world’s poorest countries by 2015 and last year approved an
additional 16 countries—12 in Africa—for rotavirus vaccine support. Last month, GAVI announced it had
secured low prices on rotavirus vaccines
from both GlaxoSmithKline (GSK) and Merck & Co. Inc. (Merck), the two
manufactures with licensed and approved rotavirus vaccines. While the first
six GAVI-eligible countries introduced GSK’s Rotarix® vaccine into their
national immunization programs, today’s introduction in Rwanda marks the
first rollout of Merck’s RotaTeq® vaccine in a GAVI-eligible country. In
developing countries such as Rwanda, where the toll of rotavirus disease is
devastating, GAVI’s support for the affordable and financially sustainable
introduction of rotavirus vaccines in national immunization programs will
make a significant impact on global efforts to achieve Millennium
Development Goal 4, the reduction of child mortality.
April 24, 2012
Rotavirus vaccines projected to save more than 2.4 million lives in developing countries by 2030 Special supplement to the journal Vaccine provides critical insights to
maximize vaccine impact in low-resource settings
Rotavirus vaccines offer the best hope for preventing severe rotavirus
disease and the deadly dehydrating diarrhea that it causes, particularly in
low-resource settings where treatment for rotavirus infection is limited or
unavailable, according to studies published in the April 2012 special
supplement to the journal Vaccine. The special supplement, “Rotavirus
Vaccines for Children in Developing Countries,” summarizes data on the
performance of rotavirus vaccines to help maximize their impact in
developing countries, which stand to experience the greatest overall public
health benefit from the introduction of rotavirus vaccines due to their
extremely high rates of severe rotavirus disease and death. Swift and significant declines in hospitalization and deaths due to
rotavirus and all causes of diarrhea have been observed in many of the 30
countries that have introduced rotavirus vaccines into their national
immunization programs to date. The studies in the supplement provide
critical insights on factors that contribute to varying efficacy of
rotavirus vaccines in different populations, and add to the growing body of
evidence demonstrating that rotavirus vaccines are a safe, proven,
cost-effective intervention. The GAVI Alliance recently secured a
new low price of US$2.50 per dose for rotavirus vaccines, which enables
GAVI to respond to developing country demand for this lifesaving intervention.
figure 1. More than 2.4 million lives saved in developing countries by 2030
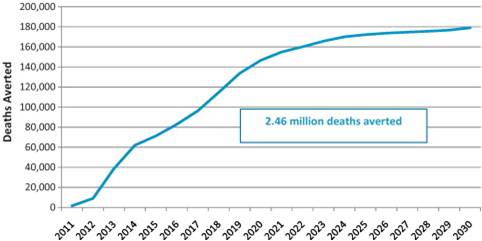
Supplement Highlights
Highlights of the findings in “Rotavirus Vaccines for Children in Developing
Countries” include:
- Rotavirus vaccines are highly cost-effective and are projected to
substantially reduce child deaths. In GAVI-eligible countries, where 95
percent of deaths due to rotavirus occur, more than 2.4 million child
deaths can be prevented by 2030 by accelerating access to lifesaving
rotavirus vaccines.
- Each year in GAVI-eligible countries, use of rotavirus vaccines
could prevent an estimated 180,000 deaths and avert 6 million clinical
and hospital visits, thereby saving US$68 million in treatment costs
annually.
- Rotavirus vaccines significantly reduce serious rotavirus disease
and save lives in rural settings, where children often die from
rotavirus infection because access to lifesaving rehydration treatment
for severe rotavirus-related diarrhea is limited or unavailable.
“Rotavirus causes more than 450,000 deaths each year in children under
five and is responsible for millions of hospitalizations and clinic visits.
A better understanding of the science and performance of rotavirus vaccines
allows developing countries, which shoulder 95 percent of the global death
toll from rotavirus, a vital opportunity to save more lives,” said Dr. Kathy
Neuzil, co-editor of the supplement and incoming Director of the Vaccine
Access and Delivery Global Program at PATH. PATH: Maximizing Impact of Rotavirus Vaccines PATH’s commitment to maximizing the impact of rotavirus vaccines in
low-resource settings and accelerating their access to children most in need
is longstanding and resolute. First under the GAVI-supported Rotavirus
Vaccine Program (RVP) (2003-2009), and now as part of the GAVI-supported
Accelerated Vaccine Introduction initiative (2009-present), and the Bill &
Melinda Gates Foundation-supported Rotavirus Vaccine Impact project, PATH
has been instrumental in the design and conduct of rigorous scientific
studies to demonstrate safety, efficacy, impact, and cost-effectiveness of
rotavirus vaccines. Dr. Neuzil, who served as Clinical Director of RVP, provides a
first-person account of PATH’s efforts to increase the scientific
evidence base to support policymaker and donor decision-making around the
acceleration of access to and delivery of rotavirus vaccines.
June 6, 2011 Substantial Price Reductions for Rotavirus Vaccines for GAVI-eligible Countries In the lead-up to GAVI’s pledging conference on June 13, 2011, multinational
and emerging market manufacturers offered steep price cuts on rotavirus
vaccines and other GAVI-supported vaccines. The substantial price reductions
coupled with the expected high number of rotavirus vaccine applications from
GAVI-eligible countries will maximize GAVI’s ability to provide life-saving
rotavirus vaccines to children in the world’s poorest countries.
GlaxoSmithKline has offered to supply up to 125 million doses of its
Rotarix® vaccine over five years to the GAVI Alliance at US$2.50/dose
($5.00/course), which is approximately a 95% reduction to the Western market
price and a 67% reduction from the lowest price available on the public
market today. Merck has offered to supply its RotaTeq® vaccine to the GAVI Alliance at $5.00/dose ($15.00/course), with
the purchase price decreasing to $3.50/dose ($10.50/course) once the
purchase volume increases to 30 million doses. Bharat
Biotech (pdf) has offered a future price to
the GAVI Alliance of $1.00/dose ($3.00/course) for ROTAVAC®, its rotavirus
vaccine currently in Phase 3 clinical trials, which is anticipated to be
ready for purchase through UNICEF by 2015. The Serum Institute of India and
Shantha Biotechnics, a subsidiary of Sanofi Pasteur, are also developing
rotavirus vaccines for GAVI-eligible countries. Part of GAVI’s 2011–2015 strategic plan includes market-shaping activities (PDF) to
minimize the cost of vaccines to GAVI and eligible countries, while
providing a sufficient and uninterrupted supply of high-quality vaccines and
fostering an environment for innovation. The reduction in vaccine prices
achieved by GAVI reflects the power of pooled procurement and predictable
financing. Helen Evans, GAVI’s interim CEO, welcomed the manufacturers’
commitment to lower prices and stated that
"if rotavirus vaccine could
be purchased this year at a $2.50 price, the impact on public health could
be significant and would allow GAVI to save approximately $500 million
through to 2020, or about $140 million through to 2015." The PATH Rotavirus Vaccine Trials Partnership is a collaboration between
PATH, the World Health Organization, the US Centers for Disease Control,
clinical study sites, and vaccine manufacturers. The partnership's
activities are funded by the GAVI Alliance. For background information on rotavirus and diarrheal disease, please see www.rotavirusvaccine.org and www.defeatDD.org. For background information on the GAVI Alliance's support for rotavirus
vaccine introduction, see www.gavialliance.org
March 23, 2010
US FDA: Evidence of porcine circovirus in rotavirus vaccine does not pose public health risk The US Food and Drug Administration (FDA) has temporarily
suspended use of the Rotarix® rotavirus vaccine, pending further
investigation of the presence of porcine circovirus within the vaccine. Porcine circovirus is commonly found in meat and other food products, and is
not known to cause disease in either humans or other animals. The FDA stressed that there is no evidence of an associated health risk. An
investigation by the European Medicines Agency
similarly concluded that there is no evidence of a public health risk,
and the World Health Organization
concurred with both the FDA and the European Medicines Agency. While rotavirus occurs worldwide, developing countries suffer the greatest burden, with more than half a million children
dying every year due to severe infections. Recent publications in the New England Journal of Medicine showed a substantial reduction in deaths of
Mexican children due to diarrheal disease after the introduction of Rotarix®, and in clinical trials in impoverished, high-mortality communities in
Africa, the vaccine significantly reduced severe rotavirus disease in African infants. Because of the tremendous burden of disease in developing
countries, the director of the US Centers for Disease Control and Prevention stressed that the known benefits of continued use of Rotarix® far outweigh a
theoretical risk of harm and encouraged its continued use in countries where rotavirus burden is acute.
February 3, 2010 Rotavirus vaccines demonstrate impact through routine use and efficacy in the developing world New
data published last week in the New England Journal of Medicine reveal the
impact of rotavirus vaccines and their lifesaving potential for the developing world. Data from Mexico, which began immunizing children against rotavirus in 2006,
illustrate the real-world impact of rotavirus vaccines when added to routine
immunization programs. During the 2009 rotavirus season, diarrheal disease death
rates dropped by more than 65 percent among children aged two years and younger.
In addition, vaccination may also have protected unimmunized children in the
same community by reducing their exposure to rotavirus. In South Africa and Malawi, a clinical trial in high-mortality, low-income
settings showed that the vaccine significantly reduced
severe rotavirus disease—by 61.2 percent—among African infants. Coordinated by
PATH's Rotavirus Vaccine Trials Partnership and vaccine manufacturer
GlaxoSmithKline, the study provided crucial data that was instrumental to
informing the World Health Organization's recent recommendation that rotavirus
vaccines be included in every country's immunization program. Data from a clinical
trial of Merck Sharp & Dohme's rotavirus vaccine,
RotaTeq®, (conducted in Bangladesh, Vietnam, Ghana, Kenya, and Mali) are expected later this year. Rotavirus vaccines can save lives when introduced in the high-burden
countries where they are most needed. In the developing world, where rates of
severe rotavirus disease are order of magnitudes higher than industrialized
countries, rotavirus vaccines can have a major public health impact. In
countries where rotavirus vaccines have already been introduced, progress is
evident, and organizations such as the GAVI Alliance and WHO are working to make
sure momentum is maintained and rotavirus vaccines soon reach all children who need them.
Watch a new video from PATH to learn more about the progress and promise of rotavirus vaccines.
January 29, 2010 PATH’s Enhanced Diarrheal Disease Control Initiative is pleased to
share with you a new video about the promise and potential of rotavirus vaccines:
http://www.path.org/media/common-disease-promising-solution.php. We are thrilled to launch this video in the midst of several exciting
developments this week. Here’s what’s causing the buzz:
- To usher in the new year, Bono, lead singer of U2 and co-founder of the ONE Campaign, published an
editorial in the New York Times
that proposed "10 ideas that might make the next 10 years more interesting, healthy, or civil."
Rotavirus vaccines make the list.
- Yesterday (Thursday, January 28), the New England Journal of Medicine published
two studies
that provide ground-breaking data for accelerating the introduction of rotavirus vaccines.
Click here
to view PATH’s press release.
- Today (Friday, January 29), at the World Economic Forum,
Bill Gates announced that he would commit $10 billion
over the next 10 years to help research, develop, and deliver vaccines to developing countries. The Gates Foundation cites
PATH's Rotavirus Vaccine Program
and the New England Journal of Medicine research
on rotavirus vaccines as examples of the encouraging progress that inspired them to commit more resources to this endeavor.
These developments make the video all the more timely as an important
resource to sustain momentum and get the vaccines to the children who
need them most. We’d be grateful if you could assist us in our outreach
by sharing the video as widely as possible with your colleagues, in your
newsletter, and on your website. If you engage in social media outreach,
we would also like to invite you to update your Facebook status with the
video link and re-tweet some of the recent announcements we've been
making on PATHtweets about rotavirus vaccines (http://twitter.com/PATHtweets).
December 3, 2009 Vaccine journal’s special edition chronicles a decade of rotavirus surveillance in Asia
A new, special edition of the journal Vaccine broadens understanding
of the tremendous burden of rotavirus disease in Asia. The Asian
Rotavirus Surveillance Network, established in 1999, worked during the
past decade to gather and disseminate rotavirus disease and strain
burden data in 23 countries/regions, 11 of which are eligible for
support from the GAVI Alliance. In addition to documenting severe rotavirus and resulting
hospitalizations in countries throughout Asia—from Kyrgyzstan, Pakistan,
and India to China, Vietnam, and the rest of the Pacific Rim region—the
edition also covers the distribution of rotavirus genotypes across the
region. Vaccine introduction efforts, including evidence of rotavirus
vaccines’ impact through routine immunization programs, are also highlighted. A commentary article, “Rotavirus vaccines: The role of researchers in
moving evidence to action,” explores how researchers apply their data to
inform policymaking, encouraging them to build partnerships with
organizations advocating for child survival and to explain the
significance of their research findings to decision-makers. To request full text of specific articles, contact
[email protected].
November 6, 2009 Journal of Infectious Diseases special edition highlights rotavirus
burden data and role of vaccines to save lives This week, the
Journal of Infectious Diseases published a special edition
highlighting crucial data collected from rotavirus surveillance networks
around the world. Expanded surveillance over the past several years has
provided important insight on rotavirus disease burden and the potential
impact of rotavirus vaccines. The tremendous burden documented by
regional surveillance networks and presented here supports the need for
widespread use of rotavirus vaccines, as recommended by the World Health Organization.
Articles in the journal supplement also cover strain diversity, the
need for continued disease surveillance, and vaccine cost-effectiveness.
Rotavirus: Every child should be vaccinated against diarrheal disease, WHO says
NY Times (6/8/09)
GAVI rotavirus
vaccine introduction support expands to Nicaragua
The GAVI Alliance
announced provision of support for rotavirus vaccine introduction to
Nicaragua, which will allow the public sector to
administer rotavirus vaccine to approximately 334,600 children. GAVI also
announced support for pneumococcal vaccine introduction in Cameroon, Congo,
and Yemen, while Armenia, Bhutan, Cambodia, Laos, and Sao Tome will receive
support for introduction of a vaccine against Haemophilus
influenzae type b (Hib). The decision follows
an October board meeting at which GAVI committed
to seek the US$3 billion necessary to continue existing programs and expand
its portfolio of new vaccine support through 2015.
Expert group
reviews data on rotavirus vaccine safety and efficacy in Africa
A recent meeting of the WHO’s Strategic Advisory
Group of Experts (SAGE) featured an update on rotavirus disease and
vaccines, including preliminary results of vaccine safety and efficacy
trials in Africa. The trials, which PATH is sponsoring in collaboration with
manufacturers GlaxoSmithKline (GSK) and Merck, will provide important data
that speak to a previous SAGE recommendation that studies be conducted in
these regions to inform a review of available data toward a global
recommendation on the use of rotavirus vaccines. SAGE will review complete
results in April 2009. A full report of the November 2008 meeting will soon
be available on the
SAGE website.
Successful trial
results could lead to global recommendation on use of rotavirus vaccines
A recent summary of global rotavirus
surveillance in the WHO’s Weekly Epidemiological Record emphasized
the importance of data on epidemiology and burden of rotavirus disease for
decision-makers considering vaccine introduction. Also essential for
building an evidence base for review by country- and global-level officials
are forthcoming data from clinical trials in Africa and Asia, which PATH is
supporting in collaboration with the vaccine manufacturers. “If they
demonstrate that the vaccine is efficacious,” states the article, “rotavirus
vaccines might soon be recommended for global use by WHO.”
Data generated through surveillance networks in all regions of the world
not only provide information on existing disease burden, but they also
provide a baseline for evaluating the future impact of routine rotavirus
vaccination. Click here
to read the full article.
Enrollment
underway for rotavirus vaccine effectiveness study in Bangladesh
In late September, investigators at the
International Centre for Diarrheal Disease Research, Bangladesh, in
partnership with RVP, GSK, the Government of Bangladesh, and UNICEF,
initiated a phase IV cluster-randomized effectiveness study of RotarixTM.
The study will evaluate the population effectiveness of RotarixTM
and its impact on reducing rates of hospitalization for acute diarrhea due
to rotavirus.
Study reveals
RotarixTM is well tolerated among HIV-positive infants in South
Africa
Results from a phase II study of the safety and
immunogenicity of RotarixTM among HIV-infected infants in South
Africa demonstrated that the vaccine was well tolerated and immunogenic when
co-administered with routine childhood vaccines. Additionally, the rotavirus
vaccine did not impact the clinical status of the HIV-positive infants.
Presented at the Interscience Conference on Antimicrobial Agents and
Chemotherapy in Washington, DC, in October, the data add to a
growing evidence base on rotavirus vaccines’ performance in Africa.
Vietnam launches
plans for renewed commitment to diarrheal disease control
PATH and the Vietnam
Ministry of Health (MOH) formally initiated an effort to strategize for
diarrheal disease control. A day-long workshop brought together officials
from a range of MOH divisions, pediatricians from national and provincial
hospitals, and representatives from PATH, UNICEF, and the Pasteur Institute.
Presentations provided an overview of the diarrheal disease burden in
Vietnam and information about interventions for diarrheal disease control,
as well as a summary of an Enhanced Diarrheal Disease Control Initiative
(EDD) project in Kenya.
Led by the Vice Minister
of Health, the MOH and PATH will establish a technical working group to
develop new national guidelines for diarrheal disease control, including the
use of zinc treatment, low-osmolarity ORS, enteric vaccines, and the
appropriate use of antibiotics. Development of the national strategy will be
informed in part by an EDD demonstration project in the central province of
Binh Dinh, where a community-based program will build awareness of
interventions and train health care workers on proper management of
diarrheal disease.
Vaccine slashes diarrheal illness in kids
Associated Press (10/25/08)
September 2008
WHO meeting on new
vaccines yields recommendations on rotavirus vaccine introduction
The annual meeting on new and underutilized vaccines implementation (NUVI),
convened by WHO, brings together global-, regional- and country-level
stakeholders representing both the private and public sectors. The 2008
meeting, held June 23–25 in Geneva, featured a discussion on introduction
issues related to rotavirus vaccines. The resulting recommendations include
the following:
- Partners should continue to advocate for political and financial commitment for new vaccine introduction.
- Solid technical and cost-effectiveness data should be the basis for country-level decisions.
- Countries should undertake effective planning prior to vaccine introduction, particularly with regard to training, cold chain capacity assessment, and vaccine supply requirements, given the markedly increased storage volumes required by the current presentations of rotavirus vaccine.
Further details on the
2008 NUVI meeting and the
NUVI web page on rotavirus
are available online.
Murdoch Children’s Research Institute collaborates with PATH to develop new
rotavirus vaccine candidate
Under a new partnership, Murdoch Children’s Research Institute (MCRI) and
PATH will support the
further development of the MCRI rotavirus vaccine candidate, RV3. PATH
will provide up to US$350,000 to assist MCRI in the production of clinical
trial lots of RV3 under Good Manufacturing Practices (GMP) at Meridian Life
Science in Memphis, Tennessee, in preparation for Phase 1 and 2 clinical
trials to be conducted by MCRI.
The RV3 vaccine candidate was developed from a strain of rotavirus that
was discovered in babies at a newborn nursery in Melbourne, Australia.
Babies who were naturally infected with the RV3 strain had no symptoms and
were protected from contracting rotavirus disease in the first three years
of life.
Enhanced diarrheal
disease control efforts expand to Asia
While studies to determine the safety and efficacy of rotavirus vaccines
in Asia are ongoing, PATH is helping to raise awareness in the region,
placing rotavirus in the context of broad diarrheal disease control
planning. A new collaboration with the Ministry of Health (MOH) in
Indonesia’s South Sulawesi province is educating health workers about
diarrheal disease control interventions and distributing updated standards
of practice. Future activities will look to expanding use of these standards
to improve clinical case management nationwide.
In Vietnam, PATH is collaborating with the MOH and other in-country
partners to evaluate the evidence for and feasibility of rotavirus vaccine
uptake, along with the potential for future enteric vaccines. A pilot
project in Binh Dinh province will scale up use of diarrheal disease
treatment interventions, including zinc and low-osmolarity oral rehydration
solution, and results will inform national planning.
Rotavirus
Surveillance News summarizes ongoing vaccine effectiveness studies
The latest issue of Rotavirus Surveillance News provides an update
on vaccine effectiveness studies in Latin America. The Pan American Health
Organization, the US Centers for Disease Control and Prevention, and the
Rotavirus Vaccine Program (RVP) are collaborating with the MOH in Nicaragua
on a case control study to evaluate the effectiveness of RotaTeq®,
manufactured by Merck & Co., Inc. These partners also are working with the
MOH in El Salvador on a similar study of Rotarix®, manufactured by
GlaxoSmithKline. Both studies were initiated in 2007 and are ongoing.
Further details are available in the
July 2008 surveillance newsletter.
In other rotavirus surveillance news,
a
recent article in Vaccine summarizes activities of the Asian
Rotavirus Surveillance Network, including measurement of disease burden in
GAVI-eligible countries and economic evaluations of the cost-effectiveness
of rotavirus vaccine introduction.
US immunization
recommendations updated to include Rotarix® vaccine
Following
FDA approval of the Rotarix® vaccine manufactured by GlaxoSmithKline,
the US Advisory Committee on Immunization Practices
updated its recommendations on rotavirus vaccination to include
information on administering the newly licensed vaccine. Data on both
Rotarix® and Merck’s RotaTeq® presented to the ACIP during its June 2008
meeting are
available online.
Survey highlights need
to elevate priority of diarrheal disease
A recent PATH research report shows that global health policymakers,
donors, and scientists are concerned that diarrheal disease does not receive
enough attention among global health priorities. The research, conducted by
FSG Social Impact Advisers, included interviews and surveys of 100
international stakeholders to gain insight into the global policy and
funding environment surrounding diarrheal disease. Respondents expressed
great interest in efforts that promote an integrated approach to diarrheal
disease control, and they identified water and sanitation, oral rehydration
solution/therapy, breastfeeding, and vaccines as the key elements that
should be included in a “package” of interventions.
Improved access to
rotavirus resources on the web
PATH’s online rotavirus resources were recently enhanced with the
redesign of the Vaccine Resource Library (VRL) and updates to the RVP
program website.
Continuing to offer high-quality, scientifically accurate documents and links,
the new VRL is easier to navigate, with a web-based database that
provides several ways to access content. The VRL is geared for health
professionals in the developing and industrialized worlds, as well as
journalists, policymakers, community leaders, parents, and anyone else
interested in vaccine-related resources.
The RVP website now offers
updates on strategic programmatic objectives to address country needs,
convene key partners, and conduct rigorous science. An interactive timeline
also depicts the many advancements in rotavirus surveillance and control,
achieved by several committed partners in just a few short years.
Editorial highlights
health-environment connection for diarrheal disease control in Africa
A recent editorial from the director of PATH’s Enteric Vaccine Initiative
emphasized the potential of diarrheal disease control in addressing
Millennium Development Goals on child health and environmental
sustainability. Marking the close of the First Inter-Ministerial Conference
on Health and the Environment in Africa,
the article calls
for action in addressing “one of Africa’s great, but ignored, health
crises.”
Mauritius: "Increasing the population's health increases its wealth"
AllAfrica.com (7/23/08)
June 2008
Rotavirus symposium presents scientific
developments with public health perspective
The 8th International
Rotavirus Symposium
brought together country leaders and scientific experts to discuss
progress and remaining challenges in making rotavirus vaccines accessible to
children around the world. The event, convened by the Albert B. Sabin
Vaccine Institute, the Fogarty International Center, the Norwegian Institute
of Public Health, the US Centers for Disease Control and Prevention (CDC),
and PATH, welcomed more than 400 participants from 67 countries—the
symposium’s highest-ever participation. Presentations provided scientific
and programmatic updates on rotavirus disease surveillance, clinical trials
to address vaccine safety and efficacy in developing countries, country
experiences with vaccine introduction, development of candidate rotavirus
vaccines, and evidence to inform country decision-making. Presentations will
soon be available on the symposium’s website.
Preliminary data reveal high efficacy of rotavirus
vaccine in South Africa
Interim results from a clinical trial of
rotavirus vaccine in South Africa and Malawi, presented
for the first time at the International Rotavirus Symposium, offer a
very promising signal about the potential of rotavirus vaccines in the
world’s poorest countries. Principal
investigator Dr. Shabir Mahdi of Dr. George Mukhari Hospital presented the
data, which reveal that Rotarix® manufactured by GlaxoSmithKline was
83 percent efficacious in preventing severe rotavirus gastroenteritis among
infants in impoverished populations of South Africa. The full results, which
will include data from children in Malawi, will be available in early 2009.
While rotavirus vaccines
are currently licensed and used in several countries, the World Health
Organization’s (WHO) Strategic Advisory Group of Experts (SAGE) recommended
clinical trials in Asia and Africa where rotavirus disease burden is very
high and where oral vaccines have the potential to perform differently. In
addition to the Rotarix® trial, PATH is conducting trials of Rotateq® in
impoverished populations of five additional countries in Africa and Asia in
partnership with manufacturer Merck & Co., Inc, with results expected in
late 2009. SAGE will review the evidence from these trials to inform its
recommendation on the global use of rotavirus vaccines.
Lessons learned in Latin America
will inform future rotavirus vaccine introduction
Rotavirus vaccine introduction in several
countries of Latin America has yielded important lessons learned, as
presented at the symposium by Dr. Lucia Helena de Oliviera, regional advisor
for new vaccines with the Pan American Health Organization (PAHO). In
particular, countries need to address the issue of cold chain storage
capacity with new rotavirus vaccines; evaluate systems to monitor
immunization coverage; train and supervise vaccine providers; and conduct
rotavirus strain surveillance. The experiences of early adopters in
considering and implementing these activities will be important for
facilitating rotavirus vaccine introduction in other countries, as well as
ensuring sustainable programs throughout the region and the world. Dr.
Oliviera and collegues further
expand upon these issues and experiences (summary
only without subscription) in a study recently published in Expert
Review of Vaccines.
Study shows rotavirus vaccines are cost-effective
in GAVI-eligible countries
Introducing rotavirus vaccines in developing
countries not only has the potential to save millions of lives, but the
intervention is also cost-effective. At the recent rotavirus symposium, PATH
senior health economist and policy officer Dr. Deborah Atherly reviewed an
analysis of the cost-effectiveness and impact of rotavirus vaccines in GAVI-eligible
countries. The analysis combined cost-effectiveness results with estimates
of vaccine adoption over time. Results indicate that rotavirus vaccination
is cost-effective in all GAVI-eligible countries and could avert the deaths
of 225,000 children per year at peak adoption and 2.5 million deaths between
2008 and 2025. In addition, cost-effectiveness ratios improve and vaccine
impact increases substantially over time, primarily due to adoption in
higher-burden countries and estimated price decline.
Dr. Atherly noted market-related issues that
must be addressed to achieve these significant outcomes. These include
improving demand forecasting to assure sustainable supply, understanding and
closely monitoring the supplier landscape to ensure that supply matches
demand, and continuing to address vaccine affordability so that countries
can support sustainable immunization programs.
Partners undertake broad range of activities to address rotavirus
research priorities
While progress continues with the currently
available rotavirus vaccines, significant efforts are underway to lay a
foundation for future candidates. Dr. Duncan Steele, PATH’s senior advisor
on diarrheal disease, discussed research priorities—as recommended by SAGE
and the WHO Global Advisory Committee on Vaccine Safety—along with the
activities PATH and partners are currently conducting to address them:
- Several countries have initiated post-marketing surveillance to
assess vaccine safety with respect to intussusception and other potential
rare adverse events;
- PATH is
working closely with emerging manufacturers in India and China to
develop the next generation of rotavirus vaccines, providing clinical
expertise and an “enabling platform” of technology support to expand the
manufacturers’ capacity; and
- Surveillance activities continue to monitor strain diversity and
genotypes in various global settings.
Partner profile: Curatio International
Foundation
Manana Khotchava, a pediatrician in Georgia,
shakes her head sadly when asked about the problem of diarrheal disease in
her country. “This is a real issue,” she says. “Many children are suffering,
but it is a struggle to get our policymakers to take action because we don’t
yet have the information to convince them.”
Underreporting and unconfirmed diagnoses of
diarrheal disease are common in Georgia, Dr. Khotchava explains. While
severe cases are reported as they come across with the health care system,
she says, those are just “the tip of the iceberg.”
In Georgia, as in other former Soviet countries,
the transition to a free-market economy spurred semi-privatization of health
care. For many Georgians, though, the introduction of private health care
has meant skyrocketing costs, hindering some from seeking care for their
children for common illnesses like diarrhea. At the same time, low pay for
providers has contributed to underreporting of diarrheal disease.
Surveillance and information systems have been
strengthened during recent years, explains Ivdity Chikovani, a program
manager at the Curatio International Foundation (CIF), a nongovernmental
organization created in 1994 to help reform
health systems in transition economies. “However, they are far from
perfect,” she explains, “and it is important to work on multiple fronts to
strengthen policy, information systems, and laboratory procedures.”
In January 2008, CIF partnered with PATH to host
a regional conference in Tbilisi, Georgia. Bringing together academics,
health care workers, and policymakers, the workshop generated significant
interest in rotavirus vaccines and broader diarrheal disease control
measures. CIF and PATH continue to work with Georgian academics, health care
providers, and the Ministry of Health to address barriers to diarrheal
disease control.
The partnership has resulted in a draft
nationwide plan on prevention and treatment interventions, and has
facilitated new diarrheal disease management guidelines. The plan aims to
facilitate education of clinicians and medical students, assist with the
introduction of the new ORS and zinc preparations on the local
pharmaceutical market, create standard diagnostic laboratory procedures to
improve the disease surveillance system, implement a system for measuring
intervention impact, and revise the national multi-year immunization plan to
set the stage for introduction of the rotavirus vaccine in the coming years.
CIF’s history of strengthening health systems in
the region, as well as increasing interest in diarrheal disease control,
gives Chikovani and her colleagues hope for progress and scale-up. “We
really hope the work we are doing in Georgia will be able to be applied to
other countries in the region as well.”
World’s leading health experts urge countries to use newly released
data to prevent the death of millions of children due to rotavirus
Yahoo News (6/4/08)
May 2008
PATH implements diarrheal disease control initiative in Kenya
PATH recently launched a
pilot program in Kenya’s Western Province aimed at building awareness for
new diarrheal disease control interventions. Interactions with provincial
and district public health officials informed the project’s planned
activities, which include workshops with parents and providers over the
coming weeks to assess current practices and knowledge gaps around diarrheal
disease control. Building on the needs identified through these workshops,
PATH will then begin training providers and educating community members on
diarrheal disease control interventions, including rotavirus vaccines, zinc
treatment, and low-osmolarity oral rehydration solution, while also
generating greater support for hand washing and exclusive breastfeeding.
Results from the project will be help develop Kenya’s National Plan for
Diarrheal Disease Control, and activities were recently endorsed by Kenya’s
Child Health Inter-agency Coordinating Committee (ICC). The ICC has
appointed a technical advisory group to monitor progress and make
recommendations.
GAVI Alliance
conference notes achievements and barriers in global childhood immunization
A recent symposium in
Barcelona highlighted the impact of immunization on saving children’s lives
in the world’s poorest countries. Policymakers, researchers, and funders
came together at the event, “Advancing Immunization in Developing Countries:
New Horizons in Children’s Health,” to review achievements, explore options
for overcoming barriers, and call for continued support for immunization in
developing countries. According to WHO estimates, GAVI programs have saved
the lives of nearly 3 million children since 2000. Among the achievements
noted was the accelerated availability of new vaccines, including rotavirus,
in developing countries that carry the greatest burden.
The event marked three
years since stakeholders signed the Barcelona Declaration toward increased
investment in current and future vaccines. Both the Government of Spain and
La Caixa, the country’s largest corporate foundation—which cosponsored the
event along with the Barcelona Centre for International Health Research—have
made considerable contributions to global childhood immunization programs,
particularly to GAVI’s International Finance Facility for Immunization.
Read the GAVI press release.
Merck updates
RotaTeq® label information
Merck & Co., Inc.,
updated the post-marketing section of its US Product Circular for RotaTeq®
to indicate the occurrence of death following intussception (a rare,
life-threatening blockage of the intestine). The US Food and Drug
Administration (FDA) and Merck agreed to update the label
to stimulate early reporting of potential adverse events and to note the
potential severity of intussusception. A report to the Vaccine Adverse
Events Reporting System (VAERS) does not mean that a causal relationship
between an event and vaccination has been established—just that the event
occurred after vaccination.
Prior to the FDA’s
approval of RotaTeq® in 2006, a clinical trial with more than 70,000
subjects found that the vaccine was not associated with an increased risk of
intussusception, or other serious adverse events, when compared to placebo.
Data presented to the CDC’s Advisory Committee on Immunization Practices
following the first year of RotaTeq® use in the US demonstrated that the
rate of intussusception in infants receiving the vaccine was not greater
than expected by chance alone, indicating that the vaccine does not pose an
elevated risk for intussusception (specific data and limitations are
presented in the slide set entitled “RotaTeq®
vaccine reports to VAERS.”) In the US, the expected background rate of
naturally-occurring intussusception in unvaccinated infants of the same age
is 18 to 43 cases per 100,000, according to the US Centers for Disease
Control and Prevention (CDC).
Because of a suspected
association between intussusception and an earlier rotavirus vaccine (RotaShield®),
the CDC and FDA are closely monitoring the safety of new rotavirus vaccines.
PATH is supporting a collaboration between the CDC, FDA, and the Pan
American Health Organization to establish active surveillance of
intussusception among vaccinated and unvaccinated children in two Latin
American countries that have introduced rotavirus vaccines.
The 8th
International Rotavirus Symposium
Registration is ongoing
for the 8th International Rotavirus Symposium, June 3 to 4, 2008, in
Istanbul, Turkey. This event will bring together public health
professionals, ministry officials, and representatives from industry and the
donor community to provide updates on clinical trials of new rotavirus
vaccines, early post-marketing data on vaccine impact and safety, issues in
vaccine policy and introduction, and other relevant topics. Simultaneous
English/Russian translation will be provided.
Registration details and
other relevant information, including a preliminary program, are available
at the
conference website.
Award recognizes
contributions of leader in global rotavirus efforts
Rotavirus expert
Dr. Roger Glass was honored at this week’s Annual Conference on Vaccine
Research, where he received the Charles Merieux Award for his extensive work
to bring rotavirus vaccines to the developing world. Currently serving as
director of the US National Institutes of Health Fogarty International
Center, Dr. Glass established the Viral Gastroenteritis Unit of the US CDC,
which has led national and global rotavirus efforts, including surveillance
and vaccine introduction, for several years.
Update on
surveillance activities in Africa
The
latest issue of the African Rotavirus Surveillance Network newsletter
provides an update on surveillance activities in participating countries and
summarizes recent training activities.
Open positions
with PATH immunization programs
At PATH we believe that
every child deserves a healthy start in life. PATH's Immunization Solutions
Strategic Program supports the integration of vaccines for disease control
in developing countries by determining disease burden; conducting clinical
trials; establishing the health economics of vaccine use; and advocating for
appropriate policies. In addition, we implement activities that improve the
accessibility of high-quality immunization services. If you'd like to help
lead our growing efforts, consider applying for one of these positions:
- Senior Scientific Advisor (position #3274)
- Program
Officer (postion #3258)
- AVI Director
(position #3252)
If you are interested in
applying for any of these positions with PATH's Immunizations Solutions
Strategic Program—visit PATH's
employment page to see the relevant job announcement and application
instructions. Help us spread the word! Forward this e-mail or let us know of
people we should reach out to.
Letter to the editor: Diarrheal disease
New York Times (5/26/08)
Rotavirus vaccine shows promise in Latin American infants
Medical News Today (4/4/08)
Rotarix® rotavirus vaccine receives US FDA approval
RotaFlash (4/4/08)
February 2008
Workshop informs diarrheal disease control planning in Eastern Europe
Child health experts and
immunization program managers from Eastern Europe and Central Asia discussed
new opportunities for controlling diarrheal disease at a two-day workshop in
Tbilisi, Georgia, sponsored by PATH in partnership with the Republic of
Georgia’s Ministry of Labor, Health, and Social Affairs and the Curatio
International Foundation. Health officials from Kyrgyzstan, Ukraine,
Uzbekistan, Armenia, Azerbaijan, Moldova, Tajikistan, and Georgia discussed
the burden of diarrheal disease in their own countries and summarized
existing control strategies. Representatives from UNICEF, the World Health
Organization (WHO), the GAVI Alliance, and PATH presented regional morbidity
and mortality data, along with evidence on zinc treatment, low-osmolarity
oral rehydration solution, and rotavirus vaccines as critical tools for an
integrated approach to enhance diarrheal disease control.
PATH is now working with
the Georgian MOH to develop a national diarrheal disease control plan, and
the workshop opened the door to future collaborations with other countries
interested in developing or updating control plans, introducing rotavirus
vaccines, or accelerating uptake of other interventions.
Advisory panel
recommends Rotarix® licensure in US
The US Advisory
Committee on Vaccines and Related Biological Products
issued a positive
recommendation this week that the US Food and Drug Administration (FDA)
approve GlaxoSmithKline’s application for licensure of Rotarix® for use in
the US.
The panel’s
recommendation followed a review of data from clinical trials on the safety
and efficacy of Rotarix®. The FDA should make its ultimate decision on
Rotarix® licensure in the US within the coming weeks. The other currently
available rotavirus vaccine, RotaTeq® manufactured by Merck & Co., Inc.,
received FDA approval in 2006.
US government
acknowledges Merck contributions to public health in Nicaragua
In recognition of an
innovative partnership with the Nicaraguan MOH to introduce RotaTeq®, the US
State Department selected Merck & Co., Inc., as a finalist for its 2007
Award for Corporate Excellence (ACE). Representatives from project partners
including the Pan American Health Organization, NicaSalud, UNICEF, the
Nicaraguan Pediatric Society, and PATH attended a ceremony to acknowledge
the nomination, held at the US embassy in Managua on February 8, 2008.
The vaccine’s launch on
October 27, 2006, made Nicaragua the first GAVI-eligible country to
introduce rotavirus vaccine and marked the first time a vaccine was
introduced in the public sector of a developing country during the same year
it was introduced in the industrialized world. Historically, it has taken up
to 15 years for new vaccines to reach the world’s poorest countries.
Announcing the 8th
International Rotavirus Symposium; Call for abstracts
The 8th International
Rotavirus Symposium will bring together public health professionals,
ministry officials, and representatives from industry and the donor
community to provide updates on clinical trials of new rotavirus vaccines,
early post-marketing data on vaccine impact and safety, issues in vaccine
policy and introduction, and other relevant topics. The symposium will be
held June 3 to 4, 2008, at the Sheraton Istanbul Maslak Hotel in Istanbul,
Turkey. Registration details and other relevant information, including a
working agenda and session abstracts, will soon be posted at http://www.rotavirus2008.com
(under construction).
The Scientific
Organizing Committee is now accepting abstracts for posters relevant to
rotavirus, rotavirus vaccines, and rotavirus vaccine introduction.
Click here for guidelines. Submissions should be sent to
mailto:[email protected].
Moldova joins European Rotavirus Surveillance Network
Representatives from
PATH and the WHO Europe regional office recently visited Moldova to
establish rotavirus surveillance and generate data for officials to consider
regarding a potential application to GAVI for vaccine introduction support.
The Children’s Infectious Disease Hospital in Chisinau will establish
sentinel surveillance, with diagnostic support from the national polio
laboratory. With the initiation of these activities, Moldova became the
seventh country in the European Rotavirus Surveillance Network, with
activities supported by the US Centers for Disease Control and funding
provided by the PATH Rotavirus Vaccine Program.
Click here to read more about the European Rotavirus Surveillance
Network.
New staff join
PATH to advance control of diarrheal disease and cervical cancer
Two key staff members
recently joined PATH to bring their experience to new initiatives. Dr.
Duncan Steele joined PATH as a technical advisor on projects to develop
vaccines against enteric diseases and rotavirus. Most recently a scientist
with the WHO Department of Immunization, Vaccines, and Biologicals, where he
served as primary liaison and advisor for PATH’s Rotavirus Vaccine Program,
Dr. Steele has worked in various fields of diarrheal disease control since
the early 1980s.
Dr. Juan José Amador
joined PATH’s Nicaragua office as Director of Technology and Health Systems
for the START-UP project, aimed at creating sustainable cervical cancer
treatment programs in low-resource countries. Prior to joining PATH, Dr.
Amador worked for the Nicaraguan MOH as National Director of Epidemiology.
His leadership was instrumental in reinvigorating diarrheal disease control
strategies, including the successful introduction of RotaTeq® in Nicaragua
in October 2006.
PATH resources
summarize diarrheal disease control interventions
A new set of fact sheets
from PATH aims to raise awareness about new and established tools to fight
rotavirus and other causes of diarrheal disease in the world's poorest
countries. Click the title below to access a fact sheet on each
intervention:
Partner Profile: Noguchi Memorial Institute for Medical Research, Ghana
 An
avid investigator of rotavirus for more than 25 years, Dr. George Armah
hopes that his own drive is reflected in the momentum of future policy
decisions on rotavirus vaccines in Africa. Such policies will be boosted by
data from critical studies including the one he directs on behalf of PATH
and Merck to evaluate RotaTeq® in Ghana. Above all, he would like to see his
team’s work ease the burden of parents traveling miles on bicycle or by foot
to seek treatment for their ailing children. Prevention with rotavirus
vaccines as could be an important solution.
An
avid investigator of rotavirus for more than 25 years, Dr. George Armah
hopes that his own drive is reflected in the momentum of future policy
decisions on rotavirus vaccines in Africa. Such policies will be boosted by
data from critical studies including the one he directs on behalf of PATH
and Merck to evaluate RotaTeq® in Ghana. Above all, he would like to see his
team’s work ease the burden of parents traveling miles on bicycle or by foot
to seek treatment for their ailing children. Prevention with rotavirus
vaccines as could be an important solution.
Dr. Armah’s initial work
with rotaviruses focused on strain identification and epidemiology,
eventually leading to his post as co-investigator and researcher on
immunogenicity studies of rotavirus vaccines manufactured by Wyeth and GSK.
In mid-2006, PATH partnered with the Noguchi Memorial Institute for Medical
Research, University of Ghana, where Dr. Armah leads a team of researchers
at the Navrongo Health Research Centre
to evaluate the safety and efficacy of RotaTeq® among infants. The
partnership allows the site to build capacity in human resources and
research skills, and in turn the investigators are contributing crucial data
to not only inform the use of rotavirus vaccines in Ghana but also to
benefit the global public health community. The trial’s enrollment is now
complete, and the site is actively participating in subject follow-up
visits. Dr. Armah expects study results by the end of 2009.
New resource to
assist countries applying for GAVI support of rotavirus vaccines
Developed by PATH in partnership with GAVI and WHO,
Introduction of rotavirus vaccine with support from the GAVI Alliance:
Information to assist the national decision-making and application process
summarizes scientific evidence and operational guidelines for introducing
rotavirus vaccines. As a supplement to
GAVI’s detailed guidelines, the document aims to support decision-making
by GAVI-eligible countries in the WHO European region and provides
information on the burden of rotavirus disease; efficacy, safety, and
cost-effectiveness of available rotavirus vaccines; eligibility criteria;
application procedures; co-financing requirements; and health system
considerations.
The information pack is also available in
Russian, and updates will be posted on GAVI’s website in accordance with
new information and GAVI decisions on eligibility in other WHO regions.
Glaxo rotavirus vaccine appears safe -- US FDA panel
Reuters (2/20/08)
December 2007
GAVI Alliance approves rotavirus vaccine
applications in Latin America
Following up on its
November 2006 pledge to subsidize rotavirus vaccines in developing
countries, the GAVI Alliance recently approved applications from Bolivia,
Guyana, and Honduras. Introduction in these countries as early as next year
brings to fruition the primary goal of GAVI’s Accelerated Development and
Introduction Plans—PATH’s Rotavirus Vaccine Program and the PneumoADIP
housed at Johns Hopkins University—toward dramatically reducing the timeline
for access to new vaccines in the poorest parts of the world. Historically,
it has taken more than 15 years for new vaccines to reach the developing
world. In 2006, that timeline was significantly reduced, with rotavirus
vaccines introduced in the US and several countries of the European Union,
as well as in Nicaragua—marking the first time ever that a vaccine was
introduced in a GAVI-eligible country in the same year that it was approved
in the industrialized world. Introduction in Bolivia, Guyana, and Honduras
will bolster this important achievement.
GAVI also announced support for pneumococcal vaccine introduction in Nicaragua, Ghana, and
Honduras, in addition to funding for a significant increase in countries
introducing Hib vaccine.
Click here to learn more.
All sites enrolling participants for PATH/Merck collaboration in Asia and
Africa
In late September, a
site in Nha Trang, Vietnam, began enrolling infants for the clinical trial
of RotaTeq®, a collaboration between PATH and Merck & Co., Inc. All sites,
which also include facilities in Mali, Kenya, Ghana, and Bangladesh have now
completed or will complete enrollment during the next couple of months, with
follow-up activities ongoing.
PATH is working in
partnership with both Merck and GSK to determine the safety and efficacy of
new rotavirus vaccines in Africa and Asia, as recommended by the WHO
Strategic Advisory Group of Experts.
Long-term efficacy data on rotavirus vaccines show continued protection
Efficacy profiles of the
rotavirus vaccines produced by Merck and GSK were recently boosted by
additional data. New RotaTeq® prescribing information reveals that the
vaccine reduced hospitalizations and emergency room (ER) visits by 100
percent for serotype G9P1A(8).
These data are based on healthcare usage by participants in the
manufacturer’s vaccine previously published Phase 3 safety and efficacy trial.
An
article in The Lancet presents data that show high
efficacy for GSK’s Rotarix® administered with routine vaccines. Over two
seasons, Rotarix® demonstrated 90 percent efficacy against severe rotavirus
among European children and 79 percent against all rotavirus infections.
This issue of The Lancet also included a
commentary on the relevance of these data for rotavirus vaccine
introduction in the developing world and the need for specific studies to
determine efficacy among infants in Africa and Asia. Please note that
these articles are available to the public, but individual registration on
The Lancet website is required.
Rotavirus Surveillance News: African Regional Network
The latest issue of
Rotavirus Surveillance News provides an update on active
surveillance activities in seven African countries. Data collected through
the network reveal important information that will be relevant for
decision-making on rotavirus vaccine introduction. Two regional reference
laboratories (based in Ghana and South Africa) support the network and
provide routine training.
WHO publishes Global Framework for Immunization Monitoring and
Surveillance
WHO recently released a
new comprehensive approach to tackle challenges in immunization monitoring
and disease surveillance. The
Global Framework for
Immunization Monitoring and Surveillance was developed by WHO and the US
CDC. It responds to the need for timely and valid epidemiological and
program information that is crucial in measuring progress towards
immunization goals and controlling vaccine-preventable diseases, including
rotavirus. An
editorial in the December 2007 issue of the WHO Bulletin outlines
the resources and guidance provided through the document, along with the
rationale for its development.