|
* _ = not tested.
However, these same measures showed dramatic differences in the
microbiologic quality of ORS between the pre-intervention and intervention periods. In the
pre-intervention period, both coliform bacteria and E. coli were routinely isolated
in large quantities from ORS samples collected from the open ORS containers. Coliform
bacteria, ranging from 1.8 X 102 to 4.1 X 108
cfu/100 ml (mean = 3.4 X
107 cfu/100 ml) were isolated from all 30 (100%) pre-intervention ORS samples.
Escherichia coli, ranging from 4 to 5.3 X 104 cfu/100 ml (mean = 6.2 X103
cfu/100
ml), was isolated from 29 (97%) of 30 pre-intervention ORS samples. In contrast, during the intervention period, no E. coli and few coliform
bacteria were detected in ORS samples. Coliform bacteria, ranging from 1 to 7.0 X 103
cfu/100 ml (mean = 3.6 X 102 cfu/100 ml) were isolated from 24 (65%) of 37
intervention ORS samples. When compared with the pre-intervention period, a significant
decrease was noted in the proportion of intervention ORS samples contaminated with
coliform bacteria (P < 0.001) and with E. coli (P < 0.001) (Table 1).
Overall, there was a five-log reduction in mean coliform bacteria counts and total
elimination of E. coli from intervention ORS samples. In the pre-intervention period, mean coliform bacterial colony counts in ORS
samples collected at 8:00 AM (mean = 2.7 X 103) were lower than mean counts in
samples collected at 4:00 PM (mean = 2.1 X 106), which were, in turn, lower than mean
colony counts in 24-hour-old samples collected at 8:00 AM the following morning (mean =
8.2 7) (P < 0.001) (Figure 2).
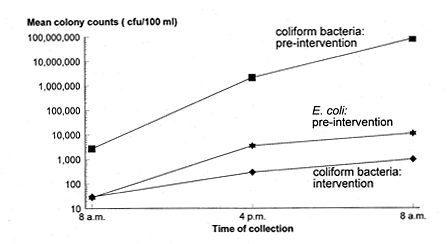 |
Figure 2. Mean coliform bacteria and
Escherichia coli colony counts in samples of oral rehydration solution on a
logarithmic scale by time of sample collection: 8:00 AM, 4:00 PM, and 8:00
AM the following morning. cfu = colony forming units
|
The same
pattern was observed for mean E. coli colony counts (2.8 X 101
versus 3.5 X
103 versus 1.1 X 104) (P < 0.01). These data suggest that either bacterial
multiplication or repeated introductions of bacteria, or both, occurred in ORS containers
on the wards during the day. In the intervention period, we a1so found a significant
difference between mean coliform bacterial colony counts in ORS samples collected at 8:00
AM, 4:00 PM, and 8:00 AM the following day (3.0 X 101 versus 2.9 x 102 versus
9.7 x 102) (P < 0.001), but no E. coli were found in any of the samples. Toxigenic V. cholerae O1, biotype El Tor, serotype Ogawa was recovered
from four (80%) of five pre-intervention ORS samples tested. All V. cholerae
isolates were resistant to tetracycline, doxycycline,
trimethoprim-sulfamethoxazole, ampillicin, and furazolidone; the same serotype and antibiogram were observed in more than
90% of the clinical isolates. No V. cholerae was found in five randomly
selected intervention ORS samples. No free or total chlorine was detected in tap water samples in either the
pre-intervention or intervention periods. During the pre-intervention period, no chlorine
was detected in any of the ORS samples 30 min after bleach had been added to prepared ORS.
During the intervention period, free chlorine levels in water samples from the vessels 30
min after bleach treatment and immediately before the addition of ORS ranged from 2.0 to
2.7 mg/L (mean = 2.5 mg/L), consistently above the World Health Organization (WHO)
recommended level of 0.5 mg/L for point-of-use water consumption. Interestingly, 30 min
after packaged oral rehydration salts were added to the chlorine-treated water, no total
or residual free chlorine was detected. Neither coliform bacteria nor E. coli were
detected in any of the packaged oral rehydration salts mixed with distilled water. At one- and two-week follow-up visits, the intervention storage vessels were
still in use, and samples of ORS from these vessels remained clean and free of
contamination with E. coli. Mean coliform bacteria colony counts in ORS samples
from 8:00 AM and 4:00 PM on these days were 1.7 X 102 cfu/100 ml at one-week and 1.0
X 104 cfu/100 ml at two-weeks follow-up. Post-intervention interviews revealed that
clinic staff and patients preferred the closed, narrow-mouth ORS storage vessels to the
old method of ORS storage and delivery from open buckets. The plastic storage vessels used in this study were manufactured in the United
States (Tolco Company, Toledo, OH), where they are sold for $6.00 each. Through an
arrangement with Rotary International and United States Agency for International
Development, similar vessels are now produced and marketed in Bolivia for approximately
$5.00 each. The cost of local commercial bleach in Guinea Bissau was $1.34 for a two-liter
bottle. Since the clinic already had bleach and ORS, the cost of the intervention was that
of the plastic vessels, a total of $20.00. Thus, most oral rehydration clinics could
easily afford this intervention.
DISCUSSION
Oral rehydration solution is a life-saving medication for dehydrating diarrheal
disease; however, it is easily contaminated when prepared in the field, and supports the
growth and survival of many types of enteropathogenic bacteria. Our study documented that
highly contaminated ORS was routinely ingested by patients on a hospital cholera ward in
Guinea-Bissau and that a simple inexpensive system for ORS preparation and storage of ORS
greatly reduced bacterial contamination. Although the hazards of ingesting contaminated ORS have never been well
documented, we believe they could be appreciable. Vibrio cholerae was isolated from
only 14 (41%) of 34 patients tested on this ward; many other patients who were
admitted for treatment of acute, watery diarrhea were likely infected with other bacterial
pathogens, such as enterotoxigenic and other diarrheogenic E, coli, Salmonella,
Shigella, and Campylobacter. We demonstrated that ORS on the ward was
contaminated with toxigenic V. cholerae that matched patient isolates, and we
suspect that it also harbored other bacterial pathogens, although we did not attempt to
identify them. By protecting ORS from contamination on the cholera ward, the risk of
nosocomial transmission of cholera and other enteric pathogens would be greatly
diminished. To improve the microbiologic safety of ORS requires that it be prepared in a
clean container from uncontaminated ingredients and that it be protected from
contamination during storage and administration to patients. The point-of-use disinfection
and safe storage system evaluated in this study address these critical control points.
Rinsing the vessels with chlorinated water before preparing ORS helps eliminate any
bacteria present from the previous day. Adding adequate hypochlorite disinfectant to water
used to prepare ORS, and waiting at least 30 min before adding the packaged salts, assures
that any bacterial contaminants remaining in the vessel or present in the water will be
inactivated before ORS is added. Finally, storing the prepared ORS in a narrow-mouth,
closed container with a spigot through which it can be dispensed provides an effective
barrier to the introduction of bacteria from contaminated hands, cups, other implements,
or insects. In our study both bleach and a special storage container were necessary to
achieve our microbiologic results. The bleach worked to avoid initial contamination of ORS
with dirty water. However, once the ORS salts were added, the bleach no longer worked, so
the effect over time of keeping bacterial contamination low may be attributed solely to
the closed container. Recognizing the risks of contamination, WHO recommends that boiled or
chlorinated water be used to prepare ORS, and that prepared ORS be discarded after 24 hr.
However, boiling the amount of water recommended for oral rehydration, as much as 10–20 liters per patient per day, is time-consuming, expensive in many developing
countries, and often impractical when fuel is scarce. 20-22
Many oral rehydration clinics have
limited or no access to centrally chlorinated water, making point-of-use chlorination the
only practical strategy. An alternate to hypochlorite solutions, potash alum, has been
used to reduce bacterial contamination of water and ORS;4,23
however, it is a less effective
disinfectant and may interfere with the physiologic properties of ORS by lowering the pH. The current WHO recommendations do not adequately address the problem of
preventing ORS contamination during storage and service to patients.20
When ORS for more
than one patient is prepared and stored in a bulk container, the risk of contamination is
high. Where feasible, clinic staff may choose to wait until a patient presents to the
clinic to prepare a fresh batch of ORS in an individual container for that patient’s
use only. Fresh ORS must be prepared again in that container each time the patient needs
more. Although this approach would limit ORS contamination from sources exterior to the
patient, and thereby reduce the risk of nosocomial transmission of enteric diseases, it
requires a sufficient supply of individual containers, and far more staff time devoted to
ORS preparation than is often available. In response to epidemic cholera, oral rehydration
treatment centers are often hastily established with minimal facilities and staff, whose
capacity to prepare or serve individual containers of ORS to each patient can quickly be
overwhelmed by an influx of acutely ill persons. Even in more permanently established
rehydration centers, the bulk preparation of ORS is often the only practical means to
assure that supply meets demand. The point-of-use disinfection and safe storage intervention we evaluated enabled
safe preparation and storage of ORS in bulk. Sodium hypochlorite disinfectant is widely
available as commercial bleach or can be generated locally through electrolysis of salt
and water.11 A standard concentration must be used to ensure that adequate free chlorine
levels are achieved in the water used for rinsing the vessels and for preparing ORS. The
hypochlorite disinfectant must be added to water at least 30 min before the addition of
packaged oral rehydration salts to ensure a bactericidal effect. The sugars in ORS, which
promote the growth of bacteria, also react with and inactivate chlorine.24
This interaction
between ORS and hypochlorite is not mentioned in most standard texts on ORS preparation.
Adding chlorine bleach to prepared ORS, as done at this clinic before the intervention, is
likely to have little effect, as the chlorine is rapidly consumed. In our field
experiment, we found that once ORS was added to bleach-treated water, residual total and
free chlorine levels rapidly decreased below detectable
levels. Since there is no residual chlorine activity in the ORS, the ORS should still be
discarded after 24 hr, even if made from chlorinated water. In areas of both endemic and epidemic diarrheal disease, closed narrow-mouth
vessels, such as those used here, and hypochlorite bleach can easily be used for
preparation, storage, and administration of ORS to reduce potential transmission of
enteric pathogens. Their application in refugee camps, where devastating outbreaks of
diarrheal diseases are commonplace, may prove particularly helpful. In any diarrheal disease treatment center that uses ORS mixed in bulk,
health-care providers should apply this intervention to prepare and provide ORS without
doing harm to the patient.
Acknowledgments:
We thank the staff of Simão-Mendes Hospital and the National
Public Health Laboratory in Guinea-Bissau, without whom this study could not have been
done; Katherine Greene and Anita Highsmith (CDC) for laboratory and technical support; and
the Millipore Foundation for generously donating laboratory equipment. Nicholas A. Daniels, Shauna L. Simons, Amabelia Rodrigues, Geir
Gunnlaugsson, Terri S. Forster, Joy G. Wells, Lori Hutwagner, Robert V.
Tauxe, and Eric D.
Mintz
Foodborne and Diarrheal Diseases Branch,
Biostatistics and Information Management Branch,
Division of Bacterial and Mycotic Diseases, and Hospital Environmental Laboratory
Branch,
Hospital Infections Program,
National Center for Infectious Diseases, and Epidemic Intelligence Service Program,
EpidemioIogy Program Office,
Centers for Disease Control and Prevention, Atlanta, Georgia
Ministry of Public Health, Bissau, Guinea-Bissau
Department of Women’s and Children’s Health,
Section for International Maternal and Child Health,
Uppsala University Hospital, Uppsala, Sweden
Authors’ addresses: Nicholas A. Daniels,
Foodborne and Diarrheal Diseases Branch,
Division of Bacterial and Mycotic Diseases,
National Center for Infectious Diseases, and Epidemic Intelligence Service,
Epidemiology Program Office,
Centers for Disease Control and Prevention,
Mailstop A-38, 1600 Clifton Road,
At1anta, GA 30333. Shauna L. Simons,
Joy G. Wells,
Robert V. Tauxe, and
Eric D. Mintz,
Foodborne and Diarrheal Diseases Branch,
Division of Bacterial and Mycotic Diseases,
Centers for Disease Control and Prevention,
Mailstop A-38, 1600 Clifton Road,
Atlanta, GA 30333. Amabelia Rodrigues,
Ministry of Public Health,
Bissau, Guinea-Bissau. Geir Gunnlaugsson,
Ministry of Public Health,
Bissau, Guinea-Bissau and Department of Women’s and Children’s Health,
Section for International Maternal and Child Health,
Uppsala University Hospital,
Uppsala, Sweden. Terri S. Forster,
Hospital Environmental Laboratory Branch,
Hospital Infections Program,
National Center for Infectious Diseases,
Centers for Disease Control and Prevention,
Atlanta, GA 30333. Lori Hutwagner,
Biostatistics and Information Management Branch,
Division of Bacterial and Mycotic Diseases,
National Center for Infectious Diseases,
Centers for Disease Control and Prevention,
Atlanta, GA 30333,
REFERENCES
1. Water with sugar and salt (editorial), 1978. Lancet 2: 300 – 301,
2. Sircar 8, Saha M, Deb 8, Deb BC, Singh PK, Pal SC, 1990. Effectiveness of oral
rehydration salt (ORS) in reduction of death during cholera epidemic. Indian J Public
Health 34: 68-70. 3. Keusch L, Keusch 6, 1984. Growth of toxigenic Escherichia coli
in oral rehydration
solutions. Diagn Microbiol Infect Dis 2: 139-143. 4. Ahmad K, Jahan K, Huq I, 1985. Decontamination of drinking water by alum for the
preparation of oral rehydration solution. Food Nutr Bull 6: 54–57. 5. Black R, Levine M, Clements M, Angle P, Robin-Browne R, 1981. Proliferation of
enteropathogens in oral rehydration solutions prepared with river water from Honduras and
Surinam. J Trop Med Hyg 84: 195–197. 6. Mathur R, Reddy V, 1983. Bacterial contamination of oral rehydration solution prepared
from well water. Indian J Med Res 78: 814–818. 7. Nagarajan L, Ganguli N, Natarajan U, Sapru S, Walia BNS, 1990. Bacterial contamination
of reconstituted oral rehydration solution. Indian Pediatr 27: 21–25. 8. Adhikari R, Rai S, Pokhrei B, Khadka JB, 1989. Comparative bacterial study of oral
rehydration solution (ORS) prepared in plain unboiled and boiled drinking water of
Kathmandu valley. Indian J Pediatr 56: 213–217. 9. Shields DS, Nations-Shields M, Hook EW, Araujo JG, Auxiliadora de Souza M, Guerrant
RL,
1981. Electrolyte/glucose concentration and bacterial contamination in home-prepared oral
rehydration solution; a field experience in northeastern Brazil. Brief Clin Lab
Observations 98: 839–841. 10. Han A, Oo K, 1989. Contamination of drinking water during collection and storage.
Trop
Geogr Med 41: 138–140. 11. Mintz E, Reiff F, Tauxe R, 1995. Safe water treatment and stor- age in the home: a
practical new strategy to prevent water-borne disease. JAMA 273: 948–953. 12. Quick RE, Venczel LV, Gonzalez 0, Mintz E, Highsmith AK, Espada A, Damiani E, Bean NH,
De Hannover EH, Tauxe RV, 1996. Narrow mouthed water storage vessels and in situ
chlorination in a Bolivian community: a simple method to improve drinking water quality.
Am J Trop Med Hyg 54: 511–516. 13. Quick R, Venczel L, Mintz E, Soleto L, Aparicio J, Gironaz M, Hutwagner L, Greene K,
Bopp C, Maloney K, Chavez D, Sobsey M, Tauxe R, 1999. Diarrhea prevention in Bolivia
through point-of-use water treatment and safe storage: a promising new strategy.
Epidemiol
Infect 122: 83–90. 14. Sobel J, Mahon 8, Mendoza C, Passaro D, Cano F, Baier K, Racioppi F, Hutwagner L,
Mintz E, 1998. Reduction of fecal contamination of street-vended beverages in Guatemala by
a simple system for water purification and storage, handwashing, and beverage storage.
Am
J Trop Med Hyg 59: 380–387. 15. Standard Methods for the Examination of Water and Waste-water, 1992. Eight edition.
Washington, DC: American Public Health Association, American Water Works Association,
Water Environment Federation. 16. Grant MA, 1997. A new membrane filtration medium for simultaneous detection and
enumeration of Escherichia coli and total coliforms. Appl Environ Microbiol 63: 3526–3530. 17. Haas N, Heller B, 1988. Averaging of TNTC counts. Appl Environ Microbiol 54: 2069–2072. 18. Fields P, Popovic T, Wachsmuth K, OIsvik 0, 1992. Use of polymerase chain reaction for
detection of toxigenic Vibrio cholerae O1 strains from the Latin American cholera epidemic,
J Clin Microbiol 30: 2118–2121. 19. Zeger SL, Liang KY, 1986. Longitudinal data analysis for discrete and continuous
outcomes. Biometrics 42: 121–130, 20. World Health Organization, 1993. Guidelines for Cholera Control: Geneva: WHO, 20–22. 21. Medecins Sans Frontieres, 1997. Refugee Health: An Approach to Emergency
Situations. London: Macmillan Education, Ltd" 166-169. 22. Gilman RH, Skillicorn P, 1985. Boiling of drinking water: can a fuel-scarce community
afford it? Bull World Health Organ 63: 157-163. 23. Oo K, Aung KS, Thida M, Khine WW, Soe MM, Aye T, 1993. Effectiveness of potash alum in
decontaminating household water, J Diarrhoeal Dis Res 1I: 172–174. 24. Pierce RC, 1978. The Aqueous Chlorination of Organic Com- pounds: Chemical Reactivity
and Effects on Environmental Quality, Ottawa: National Research Council of Canada,
Publication no. 16450.
|
